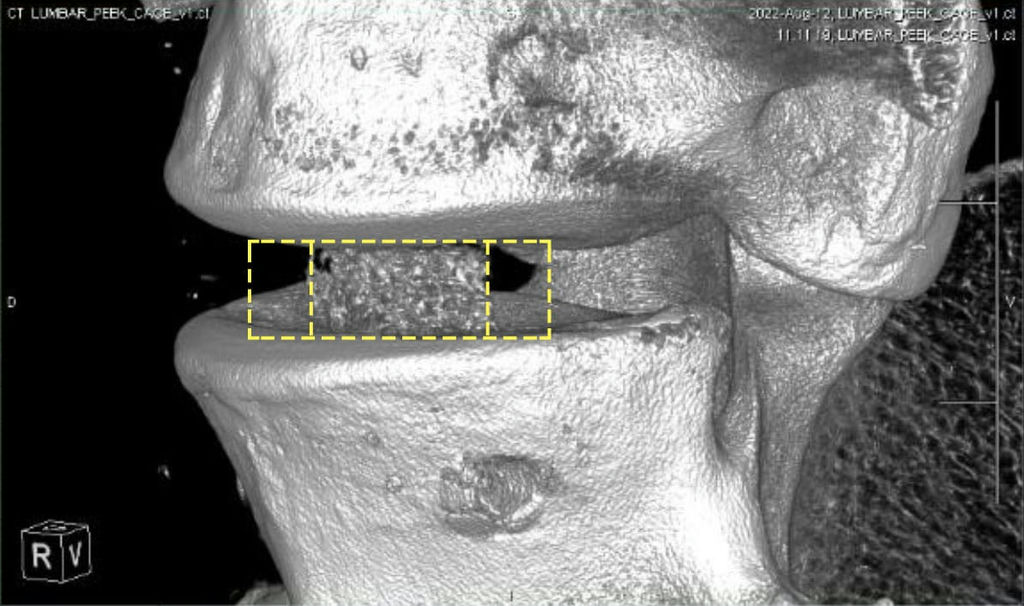
Bond3D leads the advent of the fourth generation of 3D printed PEEK polymer spinal cages which combines the advantages of all previous generations.
More from the News
Enschede, November 16th 2022 – Until recently, polymer additive manufacturing was only suitable for prototyping and simple tooling, due to the limited strength and lifetime of the printed products. In the vision of the founders of Bond3D, there would be a need to print products from high performance polymers that have high strength and are designed for longevity. Fulfilling this promise means that not just a prototype is needed for each new product, but 3D printed components can be supplied in large quantities.
Bond3D have made significant progress in maturing their technology to print high strength PEEK parts with comparable properties to PEEK components that are injection moulded or machined.
The medical device industry also uses PEEK in long term orthopaedic, spine, trauma, and cardiovascular implants. The ability of PEEK to deliver clinical and economic benefits in medical devices has led to over 15 million patients receiving PEEK implants and Bond3Ds technology will catalyze development of further solutions in medical devices by allowing the creation of high strength, porous implants to permit bone ingrowth and increase device fixation and also to allow the rapid printing of patient specific implants.
“One of the major opportunities for porous implants is in the field of spinal interbody fusion devices which are used to treat spinal problems in over a million patients each year” said HenkJan van der Pol, CTO of Bond3D.
Bond3D has partnered with Invibio Biomaterial Solutions to enable the development of the next generation of PEEK based spinal cages.
When designing an interbody fusion device the desire is to produce devices which allow the fusion to be assessed by allowing artifact-free CT/MRI images. Reducing the potential for cage subsidence by producing implants with a modulus similar to natural bone is also hugely important, in addition to including highly porous regions with the right functionality to encourage bone ingrowth. There is a need therefore to develop more advanced solutions than the current generation of porous titanium cages which have challenges in relation to imaging and stiffness.
Now is the advent of the fourth generation of spinal cages, 3D printed from PEEK, which combines the advantages of all previous generations. Bond3D has established capabilities to enable medical device companies to design highly porous PEEK cages that meet biomechanical and biocompatibility requirements needed for FDA approval.
Formnext 2022
At FormNext, HenkJan van der Pol of Bond3D and John Devine of Invibio will discuss its approach to enabling market adoption in spine in relation to:
- Proving voidlessness and high strength in PEEK parts in the elementary directions
- Testing of highly porous PEEK cages to ASTM F2027
- Producing controlled regular and complex porous structures within spinal cages
- Ensuring the reproducibility and robustness of the printers, the supply chain, documentation, and traceability.
And also how these enabling skills are being used to accelerate customers development of a arrange of new spinal devices.
Notes
Currently, the final steps towards FDA submission are being taken with a US based spine company, supported by a masterfile that will facilitate subsequent medical OEMs to bring different designs on the market.
For Bond3D, this is an ideal showcase to demonstrate that the technology is suited for use in highly critical applications in medical devices and to encourage companies to innovate with us.
Subscribe to AM Chronicle Newsletter to stay connected: https://bit.ly/3fBZ1mP
Follow us on LinkedIn: https://bit.ly/3IjhrFq
Visit for more interesting content on additive manufacturing: https://amchronicle.com
Company Press Release