The teams were led by Professor Hala Zreiqat and Dr Peter Newman at the University of Sydney’s School of Biomedical Engineering and developmental biologist Professor Patrick Tam who leads the CMRI’s Embryology Research Unit.
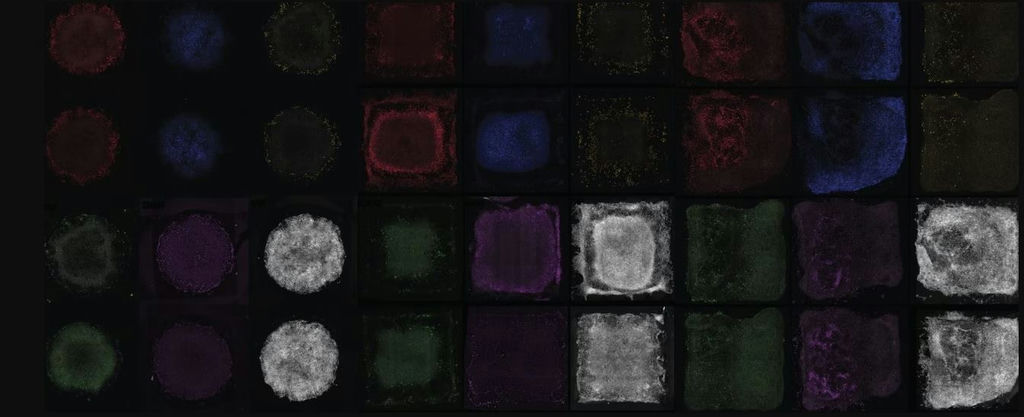
Using bioengineering and cell culture methods, the technique was used to instruct stem cells derived from blood cells or skin cells to become specialised cells that can assemble into an organ-like structure.
Similar to how the needle of a record player navigates the vinyl grooves to create music, cells use strategically positioned proteins and mechanical triggers to navigate through their intricate environment, replicating developmental processes. The team’s latest research employed microscopic mechanical and chemical signals to recreate the cellular activities during development.
Professor Hala Zreiqat said: “Our new method serves as an instruction manual for cells, allowing them to create tissues that are better organised and more closely resemble their natural counterparts. This is an important step towards being able to 3-D print working tissue and organs.”
Dr Newman said building tissues from cells required detailed instruction, not dissimilar to constructing a building from many different parts: “Imagine trying to build a Lego castle by randomly scattering the blocks on a table and hoping that they’ll fall into the correct place. Even though each block is designed to connect with others, without a clear plan, you’d likely end up with something that looks more like a large pile of disconnected Lego blocks rather than a castle.”
Seven News coverage
“The same can be said about building organs and tissues from cells: without specific instructions, the cells would likely group together unpredictably within the incorrect structures. What we’ve effectively done is create a step-by-step process that guides each building block to exactly where it should go and how it should connect with the others,” said Dr Newman.
“In line with this approach, our recently published work applies a new 3D printing method to define instructions for cells that guide them into forming more organised and accurate structures. Through this, we’ve created a bone-fat assembly that resembles the structure of bone and an assembly of tissues that resemble processes during early mammalian development.”
Research into complex tissue and organ-like structures, known as organoids, helps researchers understand how organs develop and function and how diseases affecting the organ may be caused by genetic mutations and developmental errors. The knowledge gleaned from the study also enables the development of cell and gene therapy for diseases. The ability to generate the desired cell types further provides the capacity to produce clinically relevant stem cells for therapeutic purposes.

Professor Hala Zreiqat said: “Beyond understanding the intricate ‘instruction manual’ of life, this method has immense practical implications. For instance, in regenerative medicine, where there is a pressing need for organ transplants, further research using this approach may facilitate the growth of functional tissues in the lab. Imagine a future where the waitlist for organ transplants could be drastically reduced because we can generate such tissues in the lab that sufficiently resemble their natural counterparts.”
Dr Newman said: “Moreover, this technology could revolutionise how we study and understand diseases. By creating accurate models of diseased tissues, we can observe disease progression and treatment responses in a controlled environment. We hope this could one day lead to more effective treatments and even cures for diseases that are currently hard to tackle.”
Professor Tam from CMR said: “In the past, stem cells were grown to generate many cell types, but we could not control how they differentiate and assemble in 3D.”
“With this bioengineering technology, we can now direct the stem cells to form specific cell types and organise these cells properly in time and space, thereby recapitulating the real-life development of the organ.”
The researchers are hopeful that the research will have the potential for treating vision loss caused by conditions such as macular degeneration and inherited diseases causing loss of retinal photoreceptor cells and tissue.
Professor Tam said: “If we can generate a patch of cells by bioengineering and see how the whole system functions, then we can investigate therapies that use functional cells to replace cells in the eye that were lost because of disease.”
“It would have great impact if we can deliver healthy cells into the eye. Regardless of whether the macula (the area of the retina responsible for central vision) had been lost due to inherited disease or because of trauma, the treatment would be the same.”
“The idea of treating rare genetic diseases and improving quality of life in this way is empowering. We expect that this work will lead to advanced therapies that can be moved into practice.”
The team will next focus on furthering the technique to advance the field of regenerative medicine and potentially new treatment approaches for many diseases.
Members of the research team included Hala Zreiqat, Patrick Tam, Peter Newman, Queenie Yip, Pierre Osteil, Tim Anderson, Jane Sun, Daryan Kempe, Mate Biro, and Jae-Won Shin.
Subscribe to AM Chronicle Newsletter to stay connected: https://bit.ly/3fBZ1mP
Follow us on LinkedIn: https://bit.ly/3IjhrFq
Visit for more interesting content on additive manufacturing: https://amchronicle.com