Manufacturers of 3D-printed implants, suppliers and vendors of AM systems must ensure that their products are safe and reproducible. The validation of additive manufacturing processes is the subject of a new white paper now published by TÜV SÜD.
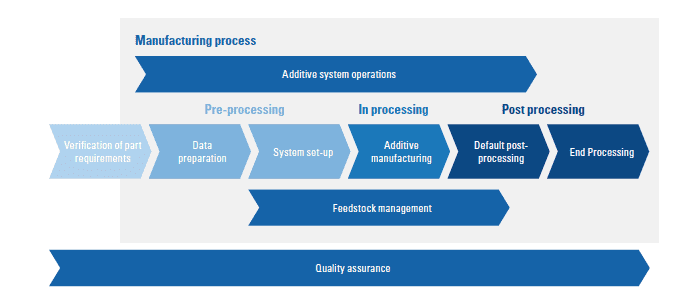
What do certification bodies or notified bodies look at when validating additive manufacturing (AM) processes? In the MedTech sector, their focus will be on personnel qualification and, in particular, the process chain. In its new white paper, TÜV SÜD outlines the state-of-the art manufacturing method and takes a closer look at the applicable standards. “We point out existing gaps and potential issues related to the additive manufacturing of medical devices, and outline feasible procedures”, says Simon Schlagintweit, Lead Auditor Additive Manufacturing at TÜV SÜD. The publication explains the individual steps in process chain qualification and validation and discuss the post-processing of products. The white paper’s target audience includes industry representatives, but also decision-makers in point-of-care diagnostics in areas such as research institutes and hospitals.
Workflow qualification and validation
Before a device destined for use in a regulated sector such as the MedTech industry can be 3D-printed, the relevant requirements need to be qualified, verified and validated. By validating their processes, companies establish that their products are in conformity with the applicable requirements. To this end, all process parameters need to be verified and observed. TÜV SÜD differentiates between two stages of validation, a conceptual and a hands-on stage. “Risk assessment is a central step of the conceptual stage“, explains Simon Schlagintweit. “It helps companies to avoid process failures and failure or contamination of components.” By mapping the process, this step identifies, evaluates and mitigates risks, relying on well-established tools such as failure mode and effects analysis (FMEA), Ishikawa diagrams (also known as fishbone diagrams) and fault-tree analyses. The hands-on stage covers the requirements related to installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ).
Applicability of the relevant standards
The validation process assesses the entire workflow in accordance with ISO/ASTM 52920, the international standard on additive manufacturing. This standard for quality management systems (QMS) in additive manufacturing is based on specification DIN SPEC 17071. By contrast, applicability of the two AM standards, ISO/ASTM 52904 and 52930 is limited; while ISO 52904 offers guidance for the use of AM in critical applications, ISO 52930 outlines general qualification principles for AM equipment. However, both standards refer to only one class of materials and/or production technology, and are thus not sufficient for qualification.
Post-processing of AM parts
Post-processing activities such as cleaning, sterilising and packaging can critically influence the biological safety, surface quality and mechanical properties of products. Given this, these activities are of crucial importance in the manufacturing of medical devices. Test requirements vary depending on the individual device and application. Biological safety requirements are summarised in the ISO 10993 standard. The requirements for packaging and sterilisation are outlined in the ISO 11607 or EN 556 standards respectively.
Personnel qualifications
Although automation is making strides, the key steps in additive manufacturing are still carried out manually. Quality management systems (QMS), such as ISO 9001, ISO 9100 and ISO 13485, thus require appropriately qualified staff. This applies to areas such as quality testing and development but also machine operators. Validation requires an expert team with expertise from all sub-areas of AM. “Competent personnel are the key to industry-grade AM production sites. AM-specific roles must be defined and trained”, emphasises Simon Schlagintweit.
The English-language whitepaper is available for free download at: https://www.tuvsud.com/en/resource-centre/white-papers/additive-manufacturing-validating-processes-to-meet-medical-regulations