A team of researchers from the Harvard Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS) has made a breakthrough in 3D bioprinting which could overcome a significant challenge in the field related to lack of cellular density for functional organs. That is, the researchers have pioneered a new bioprinting process called SWIFT (sacrificial writing into functional tissue) which is capable of 3D printing vascular channels into living matrices made up of stem-cell-derived organ building blocks (OBBs), resulting in organ-specific tissues that have high cell density and function.
The innovative approach, described as “an entirely new paradigm for tissue fabrication,” consists of a two-step process that first forms hundreds of thousands of stem-cell-derived aggregates into a dense, living matrix of OBBs. According to the research team, this dense matrix contains roughly 200 million cells per milliliter. In the second step, a vascular network is embedded into the matrix by writing and removing a sacrificial ink. The resulting vascular network allows for oxygen and other vital nutrients to reach the cells in the matrix, keeping them alive.
Co-first author Mark Skylar-Scott, Ph.D., a Research Associate at the Wyss Institute, explained: “Rather than trying to 3D print an entire organ’s worth of cells, SWIFT focuses on only printing the vessels necessary to support a living tissue construct that contains large quantities of OBBs, which may ultimately be used therapeutically to repair and replace human organs with lab-grown versions containing patients’ own cells.”
“Forming a dense matrix from these OBBs kills two birds with one stone,” added co-first author Sébastien Uzel, Ph.D., a Research Associate at the Wyss Institute and SEAS. “Not only does it achieve a high cellular density akin to that of human organs, but the matrix’s viscosity also enables printing of a pervasive network of perfusable channels within it to mimic the blood vessels that support human organs.”
In the emerging bioprinting field, researchers across the world are trying to tackle the issue of vascularization—which is necessary for keeping lab-grown tissues and cells alive. If bioprinted tissues and organs are to be implanted into humans, this is a critical step that must be achieved. SWIFT could offer a novel solution.
SWIFT uses cellular aggregates that are derived from adult induced pluripotent stem cells and are combined with a tailored extracellular matrix (ECM) solution to create a living matrix that is compacted using centrifugation (a method that uses rapid spinning to separate particles by size, shape or density). The researchers say the dense matrix has the consistency of mayonnaise when it is subjected to cold temperatures (0-4 °C), enabling it to be manipulated without losing its shape.

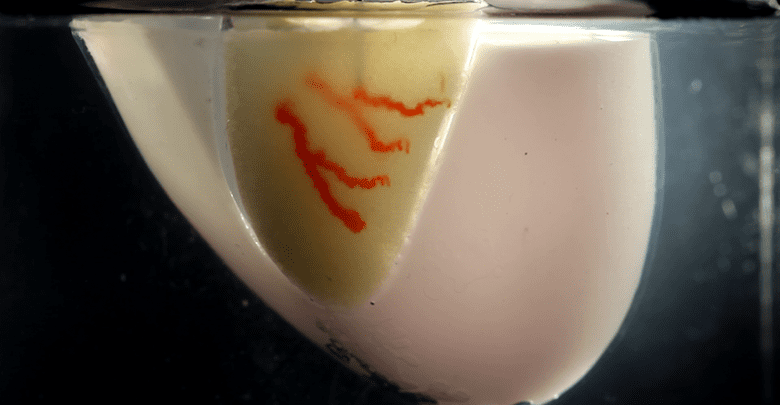
This consistency allows the researchers to employ sacrificial 3D printing, which uses a thin nozzle to deposit a strand of gelatin-based ink inside the matrix without damaging any cells. The ink is deposited until a network of blood vessel-like channels are created in the tissue. The matrix is then heated to 37 °C, causing the cells to stiffen (like an omelet) and the gelatin ink to melt away. The result is a dense cellular matrix with hollow channels embedded into it.
Eventually, the channels—which vary in size from 400 micrometers to 1 millimeter in diameter—can be perfused with oxygenated media that nourish the cells and keep the tissue alive. In tests, the tissues which had the embedded channels survived well compared to those without (which saw cell death within 12 hours).
The researchers were also successful in producing organ-specific tissues using the SWIFT process. According to a recent study published in Science Advances, the team printed a vascular network into a matrix consisting of heart-derived cells and perfused it with nutrients for a week. By the end of the week, the cardiac OBBs had fused together and formed a more solid tissue with increasingly synchronous and strong contractions—not unlike a real human heart.

“Our SWIFT biomanufacturing method is highly effective at creating organ-specific tissues at scale from OBBs ranging from aggregates of primary cells to stem-cell-derived organoids,” said corresponding author Jennifer Lewis, Sc.D., a Core Faculty Member at the Wyss Institute and the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS. “By integrating recent advances from stem-cell researchers with the bioprinting methods developed by my lab, we believe SWIFT will greatly advance the field of organ engineering around the world.”
Source: 3dprintingmedia.network