Global Advanced Metals (USA) and Croom Medical (Ireland) are collaborating to establish a turnkey tantalum-based additive manufacturing (AM) platform.
A laser powder bed fusion GE M2 Series 5 printer has been installed within Croom’s facility, with the printer being fully dedicated to the use of GAM’s tantalum AM powder. With Croom’s years of successful contract manufacturing experience, the tantalum print capability is being offered by Croom as a contract manufacturing solution, bringing the advantages of tantalum to the medical device, aerospace, and related markets.
Global Advanced Metals (GAM), headquartered in Massachusetts, USA, has more than a 70-year history of innovation and manufacturing of high-grade tantalum powders for use in electronics and other applications. GAM has more recently developed a spheriodized tantalum powder, a necessary form for use in “printing” of tantalum parts.
Based in County Limerick, Ireland, Croom Medical (Croom) has operated for more than 35 years providing contract manufacturing services to medical device (and other) OEMs. In recent years, Croom has developed an extensive capability in AM technology in collaboration with a number of academic and state-sponsored institutions and has successfully manufactured more than 20,000 devices by AM technology.
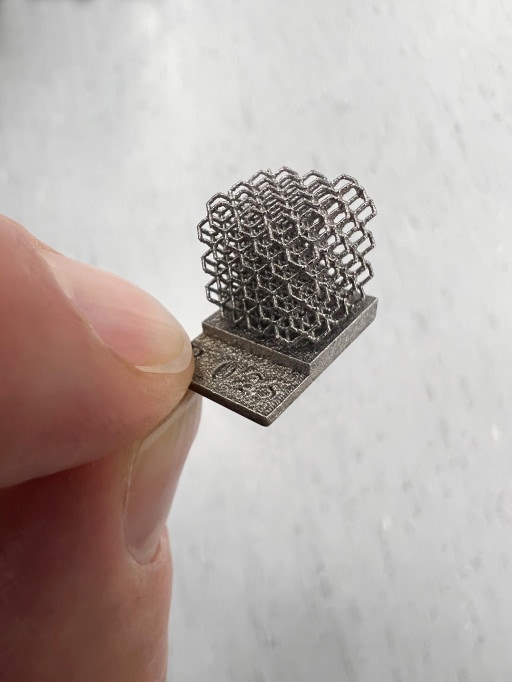
AM has a number of key advantages over traditional subtractive manufacturing processes. AM intrinsically uses less material than subtractive methods, produces more complex and lightweight part geometries, can be used for rapid prototyping, and manufactured parts can be ultimately printed in close proximity to point of use.
GAM and Croom are initially targeting the contract manufacturing of medical implants and components that would benefit from the enhanced performance offered by tantalum. Multiple papers cite advantages of tantalum-based implants compared to existing implant materials, including improved biocompatibility (reduced clotting), enhanced bone in-growth, tolerable to higher stress, and produces high surface friction promoting implant stability1,2,3,4,5. These benefits should lower the rate of surgery revisions, thus avoiding patient discomfort and unnecessary added surgery costs. Although use of tantalum in AM applications is relatively new, tantalum has been extensively and successfully used for many years in in-vivo surgical devices such as surgical staples, dental implants, and hip and knee implants. GAM and Croom believe that using AM technology to develop these devices will enable production of complex, porous, strong and lightweight structures.
As part of this collaboration, GAM will provide spherical tantalum powder, produced in its Boyertown, Pennsylvania, plant and derived from ethically and conflict-free-sourced tantalum raw material. The team at Croom will manufacture devices and components for customers using a recently acquired GE M2 printer 100% dedicated to the project and material. In a circular manufacturing cycle, tantalum raw material that cannot be reused in this AM process will be recycled back to GAM’s Pennsylvania facility. GAM’s raw material and tantalum powder expertise, Croom’s decades-long experience manufacturing, finishing and testing devices to medical device standards, and Croom’s proximity and relationship with many of the top device OEMs situated in Ireland, positions this collaboration for long-term success.
Andrew O’Donovan, CEO of Global Advanced Metals, added, “GAM is thrilled to work with such an experienced and trusted company as Croom Medical. In recent years, GAM has worked to enhance the production of ultra-pure spherical tantalum powder for 3D printing. GAM views the intrinsic purity and properties of tantalum as a wonderful fit for next generation implants and Croom is the company with the experience, capability and long-established customer relationships to bring this to market.”
1. Matsuno H, Yokoyama A, Watari F, Motohiro U, Kawasaki T., Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials. 2001 ;22:1253-1262.
2. Miyazaki T, et.al., Biomaterials. 2002;23:827-32; Balla V, et.al., Acta Biomater. 2009
3. Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000; 52:631-638
4. Hacking S, et.al., J Biomed Mater Res. 2000;52:631-638; Balla, V., et.al., Acta Biomater. 2010 Aug; 6(8): 3349-3359; Levine BR, Sporer S, Poggie RA, Valle CJD, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006; 27:4671-4681.
5. Sungail, C., Abid, A., Metal Powder Report, online 25 March 2019
Subscribe to AM Chronicle Newsletter to stay connected: https://bit.ly/3fBZ1mP
Follow us on LinkedIn: https://bit.ly/3IjhrFq
Visit for more interesting content on additive manufacturing: https://amchronicle.com