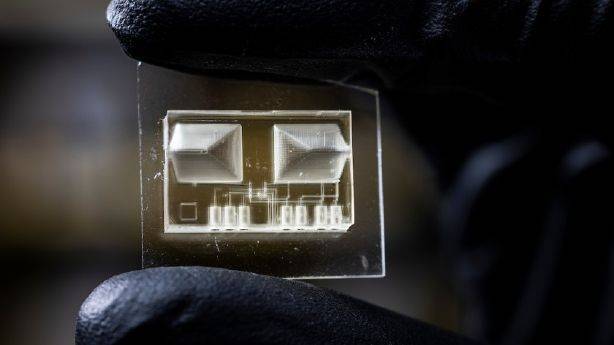
An interdisciplinary team of researchers at Brigham Young University, led by BYU engineering professor Greg Nordin, has developed a new 3D printing technique to create the smallest ever, high resolution microfluidic lab-on-a-chip devices.
Lab-on-a-chip devices are miniaturized devices that can perform analysis usually done in a lab, like biomedical diagnostics or DNA analysis. Basically, the concept is that scientists take a biomedical lab and the diagnostic equipment that would be there and shrink it down into a tiny chip that can process and measure as a lab would.
Researchers detailed the process is a new paper published in Nature Communications.
This technology has been in development since the late 1980s and has improved cost efficiency, speed, sensitivity and consistency of biochemical detection and biomedical diagnostics. Researchers say the device can also reduce human error with automated tech, allow for more controlled testing and only require minimal fluid samples.
Developers have used microfluidic technology to create microscopic channels, pumps and valves that enable tests and reactions on picometers of liquid, like a tiny portion of single drop of blood.
As far as the tech has come in the past couple of decades, one of the biggest challenges with lab-on-a-chip devices is that they are generally fabricated in clean rooms, a lab that’s free from dust and other contaminants. This process can take up to three to four days, which means that production and distribution to the market become expensive, slow and difficult.
Commercial 3D printers are not advanced enough to make the microchannels and minuscule tech for the chip. Because of this, Nordin and a team of researchers say they have built their own 3D printers for around $100,000 each that are capable of fabricating the smallest lab-on-a-chip devices yet out of a type of plastic create by photo-polymerized liquid turned into high-res, solid material layer by layer in a matter of five to seven minutes without the multimillion-dollar expense of setting up and using a clean room.
These printers only require nominal maintenance and can create valves that are only 15 microns in size.
“That’s where the real innovation is,” Nordin said, adding that because the prototypes of these devices are developed so quickly, they can take “a fail-fast-and-often approach to iterating a successful device,” whereas in a clean room each prototype takes so long to develop that it becomes “precious.”
He and his team have three 3D printers of different generations and iterations operating right now and four brand new developments in the works. They are using this technique to create a whole series of tiny pumps and valves and reaction chambers and mixtures — all on a single chip.
The low cost of development, after the initial cost of building the printer, would mean that these devices could be fabricated at a low cost, making them more available to underserved communities, he said. Their small size allows for easier handling and could be administered by a nurse, not to mention easy to distribute. It also makes developing different technologies and functions within the chip easier because prototypes are cheaper and easier to build and each chip will be able to perform multiple tests and serve multiple functions.
“This development revolutionizes (the field) in the following sense: Up to now, you don’t see many commercial microfluidic devices. Through 3D printing, we can create prototypes really quickly and the manufacturing path exactly the same as the prototyping path. Bottom line: The amount of friction is so reduced that it can really be revolutionary in developing devices,” Nordin said.
He gave an example that he and one of his colleagues, William Pitt, a chemical engineering professor at BYU, are working on submitting to the National Institute of Health. It involves significantly speeding up the treatment of sepsis, a bacterial infection that is often fatal and resistant to antibiotics. Regular biomedical testing can take days between blood draws to determine which antibiotics will be effective. Using a lab-on-a-chip device instead would shrink that time down to around two hours.
“Mortality rates completely change if you can treat a person that quickly,” Nordin said.
He believes that this new technique could completely change the way medical professionals run diagnostics and potentially save lives. He also wants Utahns to know that “this cutting-edge work going on at Utah universities is truly having an immense real-world impact and the education students get by being involved in that is really fantastic.”
Within his team, the faculty guides and directs the research and development, but the students do most of the hands-on work. The first author listed on the paper that was published in Nature Communication is a master’s student from Peru who came up with a lot of the innovative ideas, Nordin explained.
“Everyone had a role to play, and the really nice thing about it is that companies spin out of this and provide employment opportunities for high-tech employment in Utah, which is always one of our goals,” he said.