Anymedi Solution said its patient-customized surgical device that guides the resection plan during breast-conserving surgery has won innovative medical technology designation from the National Evidence-based Healthcare Collaboration Agency under the Ministry of Health and Welfare.
The company’s breast conservation surgery guide, researched and developed in collaboration with Asan Medical Center, analyzes the medical images of breast cancer patients in 3D and uses 3D printing to produce a surgical guide to remove the tumor.
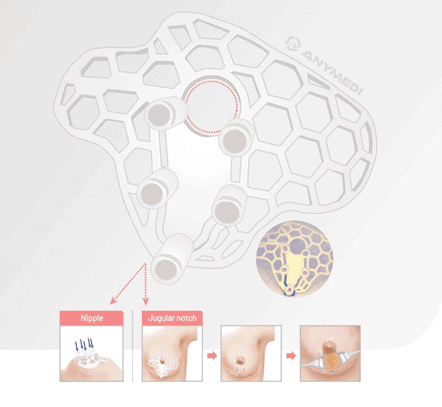
“Through this procedure, it is possible to make a more precise surgical plan and receive detailed surgery that preserves the breast as much as possible,” the company said.
The method also allows safe surgery and lowers the recurrence rate for patients undergoing chemotherapy as it effectively delivers the residual tumor suspected area invisible on ultrasound or magnetic resonance imaging (MRI) to doctors and helps minimize nipple retraction and scarring after surgery, it added.
The company stressed that the innovative medical technology for its 3D printing technology is the first for a 3D medical device in Korea.
The incidence of breast cancer is rapidly increasing worldwide. According to the National Cancer Information Center statistics, it is the most common cancer among women, it noted. Therefore, interest in effective and safe surgical methods and early diagnosis is very high. The company expects that this innovative medical technology designation will enable national support for breast cancer surgery.
“The reason the government has recognized our device as an innovative medical technology is it has achieved something that was not possible with the existing tumor-marking methods,” Anymedi CEO Kim Guk-bae said. “It is of great significance that the device can mark tumor area in MRI directly on the breast.”
He added that the technology also allows hospitals to mark tumor areas that were not visible in ultrasound or MRI in the past.
Kim stressed that the company is actively striving to enter overseas markets. In addition to the innovative medical technology recognition, Anymedi obtained certifications from the U.S. Food and Drug Administration and Europe’s Conformité Européenne in July last year and is applying for approval to Victorian Breast and Oncology Care in Australia.


