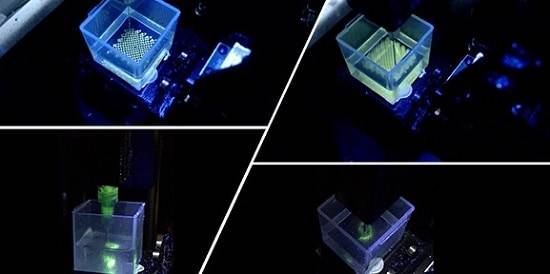
A team of engineers and clinicians have used 3D printing to create intricate replicas of human cochleae – the spiral-shaped hollow bone of the auditory inner ear – and combined it with machine learning to advance clinical predictions of ‘current spread’ inside the ear for cochlear implant (CI) patients.
‘Current spread’ or electrical stimulus spread, as it is also known, affects CI performance and leads to ‘blurred’ hearing for users, but no adequate testing models have existed for replicating the problem in human cochleae – until now.
CIs have transformed the lives of hundreds of thousands of people who suffer from severe or profound hearing loss. However, the efficacy of the surgically implanted medical device has been hampered by ‘current spread’ – a phenomenon caused by the high electrical conductivity of the fluids that sit inside cochlear ducts. These fluids prevent the CI user’s auditory nerve from being stimulated accurately, leaving many users experiencing significant distortions to the sounds they perceive.
The size and shape of a human cochlea is unique to each individual and varies from person to person. Coupled with its tricky location and complex anatomy, this makes it one of the most difficult tissues to study and thus, analysis of the ongoing problem of ‘current spread’ has not been easily possible.
Now a cross-disciplinary research team1 have been able to analyse the electric field imaging data of cochlear implant patients, thanks to 3D printed cochleae with tuneable electro-anatomy. The 3D printed models allow the researchers to study how the cochlear shape and electrical property of the biomimetic cochleae affects ‘current spread’ measured in electric field imaging. Once combined with machine learning, this co-modelling method can predict the ‘current spread’ in cochlear implant users and for the first time, extrapolate the range of patient cochlear tissue resistivity. The results are reported in the journal Nature Communications.
Ms Iek Man Lei, primary author of the study and a PhD student from the Biointerface Research Group at Cambridge, said: “Our library of 3D printed cochlear models capture the range of geometries that human cochlear lumens can take and are designed with electro-mimetic bone matrices that replicate realistic bone resistivities. The 3D printed cochlear models also have suitable mechanical property for multiple CI electrode insertion. Our ‘print-and-learn’ co-modelling concept can help decipher how different characteristics of a patient’s cochlea affect the stimulus spread.”
Professor Manohar Bance, Professor of Otology and Skull Base Surgery, from Addenbrooke’s Hospital, said: “The next steps are to use this co-modelling approach to understand patient electrical field imaging in different disease states e.g. otosclerosis (a rare condition that causes hearing loss). This will help us to understand if there are different cochlear conductivities in disease states, which could lead to unwanted stimulation of other structures such as the facial nerve. It will also help us diagnose patients who are experiencing unusual sound sensations to try to understand if their conductive pathways are different. In the bigger picture, we plan to go on from here to try to print electrically analogue whole heads to understand current pathways from implant to ground electrode on the implant, in order to better mimic the in vivo condition.”
Dr Shery Huang, Associate Professor in Bioengineering and Group Leader of the Biointerface Research Group at Cambridge, said: “We are all aware of the tremendous power of artificial intelligence (AI), but AI needs to start its learning from a good set of data. Because of patient privacy, patient safety and ethical concerns, holistically documented and characterised clinical data are hard to come by. Therefore, 3D printing is a powerful tool to create physical models which might provide a well-characterised training dataset as a purpose-built surrogate to clinical data for machine learning. The co-modelling principle demonstrated in this study could be useful to address other areas of clinical modelling and healthcare applications.”
This work was supported by the European Research Council, the Cambridge Hearing Trust and the Evelyn Trust.