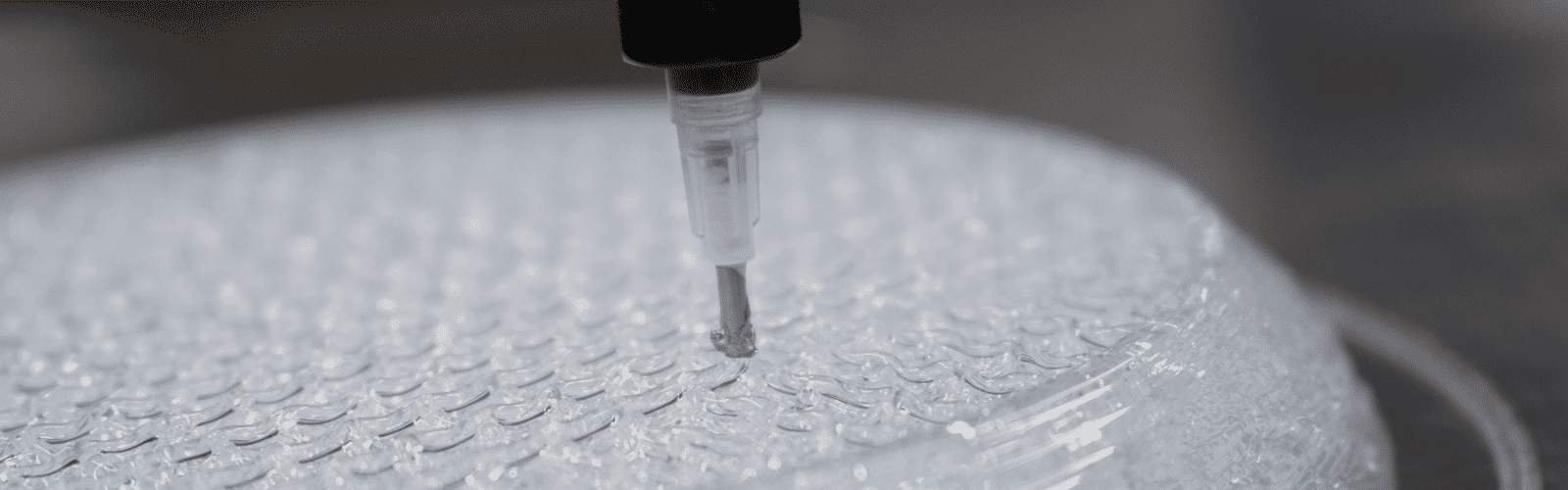
While metal Additive Manufacturing (AM) is already becoming a practice in hard implants such as bone and dental, penetration of AM still remains negligible in soft tissue implants. 3D Printing technologies for elastomers and other biomaterials are in a nascent stage, simply because of the inherent complexity of these materials. One such elastomer, silicone, is among the best-known materials when it comes to biocompatibility and safety, especially if the application requires long term implantation within the body.
Challenges in 3D printing silicone
Conventional AM materials like metals and thermoplastics can be melted and solidified using thermal triggers and this can be done any number of times. Silicone on the other hand relies on chemical crosslinking to change its form from liquid to solid and this can be done only once. While metals and thermoplastics can be drawn into filaments or ground into fine powders for 3D printing, silicone remains mostly in its messy and viscoelastic fluid form. Factors like the complex rheology or flow behaviour and curing kinetics further add limitations for conventional AM processes to deal with silicone.
One is left with only two choices, the first one is to manipulate silicone chemistry so that it can work with established AM processes, for example SLA and DLP using optical triggers for photocurable silicone. Or the other choice is to reinvent the AM process for existing silicone formulations.
At Prayasta, we are pursuing the second approach in which we have developed a dedicated AM process for implantable silicone which we call Elastomer Additive Manufacturing or EAM for short. Implantable silicone is a grade of silicone which has the highest purity and passes through numerous quality controls enforced by biocompatibility standards. Implantable silicone must not be confused with other medical grade materials that are suitable only for either a short term implantation or for externally used devices coming in contact with skin. Though the EAM process is optimized for implantable grade, other grades of silicone and elastomers can also be 3D printed using it.
We are using EAM to bring personalization to breast implants and prostheses and provide a one-to-one fitting solution for breast reconstruction, especially for post mastectomy needs where a woman has lost one of her breasts to cancer. Our goal is not limited to only provide personalization in terms of shape and size. And that is why, we have also created novel design approaches tailored for achieving personalization in terms of weight, touch and feel. Together, EAM and these design principles form a technology bundle that can be extended to other soft tissue applications in the future.
Regulatory advantages with EAM
Implantable grade of silicone has proven its reputation over several decades in terms of biocompatibility and safety. Having made a choice to develop an AM process for implantable grade has its own advantages when it comes to passing through the regulatory checks. Breast implants are class III or equivalent medical devices and require intensive in-vitroand in-vivo testing before they can be implanted in humans. Since the same grade of silicone is already used in existing breast implants and we believe that EAM does not alter the chemistry of silicone, we are expecting to achieve regulatory approvals faster, shortening our time-to-market.
However, there are still many unknowns that have to be identified that are being explored by the material experts and clinicians throughout the world. Although, adoption rate of AM in silicone implants is slow, demand for personalization is growing rapidly.
To get a deeper insight on Additive Manufacturing of Implant Grade Elastomers do tune in to my episode on AM Infocast.