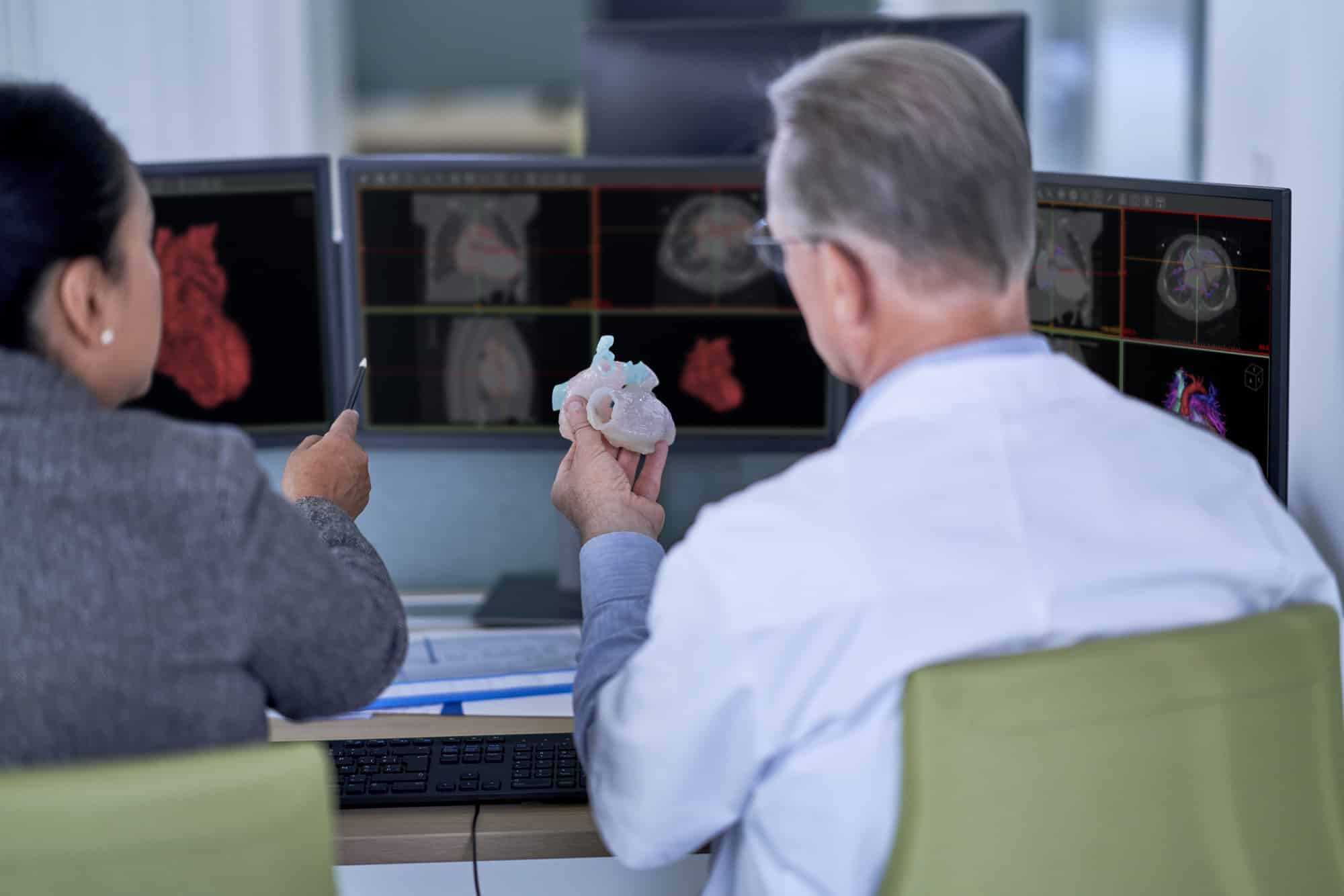
Materialise has announced that The Central Drugs Standard Control Organization (CDSCO) under Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India has licensed the medical use of 3D planning software Mimics Medical, 3-matic Medical, Mimics inPrint and ProPlan CMFTM. The medical use of the software can help surgeons to simulate and evaluate surgical treatment options before entering the operating room. The import license is another milestone for the medical 3D printing industry: patient-specific solutions are gaining ground across the world thanks to the possibilities offered by high-quality 3D planning software.
The image-processing software Mimics Medical, 3-matic Medical, Mimics inPrint and PROPLAN CMFTM have been designed to help researchers, engineers, and surgeons create accurate 3D models, design medical devices that fit each patient’s unique anatomy, and plan procedures preoperatively.
Mimics Medical and 3-matic Medical takes a surgeon seamlessly from scan to virtual surgery, taking away the guesswork. Surgeons can virtually plan a procedure, perform dedicated anatomical analysis, create accurate virtual 3D models and design patient specific devices that can help them achieve the desired patient outcomes. Moreover, the software can be implemented as an educative tool to train future surgeons.
The Mimics inPrint convert medical image data (DICOM files) to 3D models which can be used for the fabrication of physical replicas of the 3D model using additive manufacturing methods. The anatomical model can be used for diagnostic purposes in the field of orthopedic, maxillofacial and cardiovascular applications.
The PROPLAN CMFTM software is used as a preoperative tool for simulating and evaluating implant placement and surgical treatment options for cranio-maxillofacial surgeries. It allows a surgeon to use an interactive 3D rendering of the CT or MRI images for his presurgical planning.
Global increase of patient-specific solutions
The medical clearance of the 3D planning software by the India’s Central Drugs Standard Control Organization (CDSCO) aligns with the global increase of patient-specific solutions and will support the adoption of 3D planning and printing for medical use.
“Thanks to this medical clearance, our 3D planning software will no longer be limited to R&D purposes. We can now help Indian surgeons to offer their patients personalized solutions that fit their unique anatomy”, says Brigitte de Vet, Vice President Medical of Materialise.
“Moreover, the medical clearance of both Materialise software serve as a benchmark for the clinical implementation of 3D printing for physicians creating 3D models at the point-of-care.”
About Materialise
Materialise brings almost three decades of 3D printing experience to a range of software solutions and 3D printing services, which together form the backbone of the 3D printing industry. Materialise’s open and flexible solutions enable players in a wide variety of industries, including healthcare, automotive, aerospace, art and design, and consumer goods, to build innovative 3D printing applications that aim to make the world a better and healthier place. Headquartered in Belgium, with branches worldwide, Materialise combines the largest group of software developers in the industry with one of the largest 3D printing facilities in the world. For additional information, please visit: www.materialise.com.
About Materialise Medical
Materialise Medical, which has pioneered many of the leading medical applications of 3D Printing, enables researchers, engineers and clinicians to revolutionize innovative patient-specific care. Materialise Medical’s open and flexible platform of software and services, forms the foundation of certified Medical 3D Printing, in clinical as well as research environments, offering virtual planning software tools, 3D-printed anatomical models, and patient-specific surgical guides and implants. For additional information, please visit: materialise.com/medical
Subscribe to AM Chronicle Newsletter to stay connected: https://bit.ly/3fBZ1mP
Follow us on LinkedIn: https://bit.ly/3IjhrFq
Visit for more interesting content on additive manufacturing: https://amchronicle.com