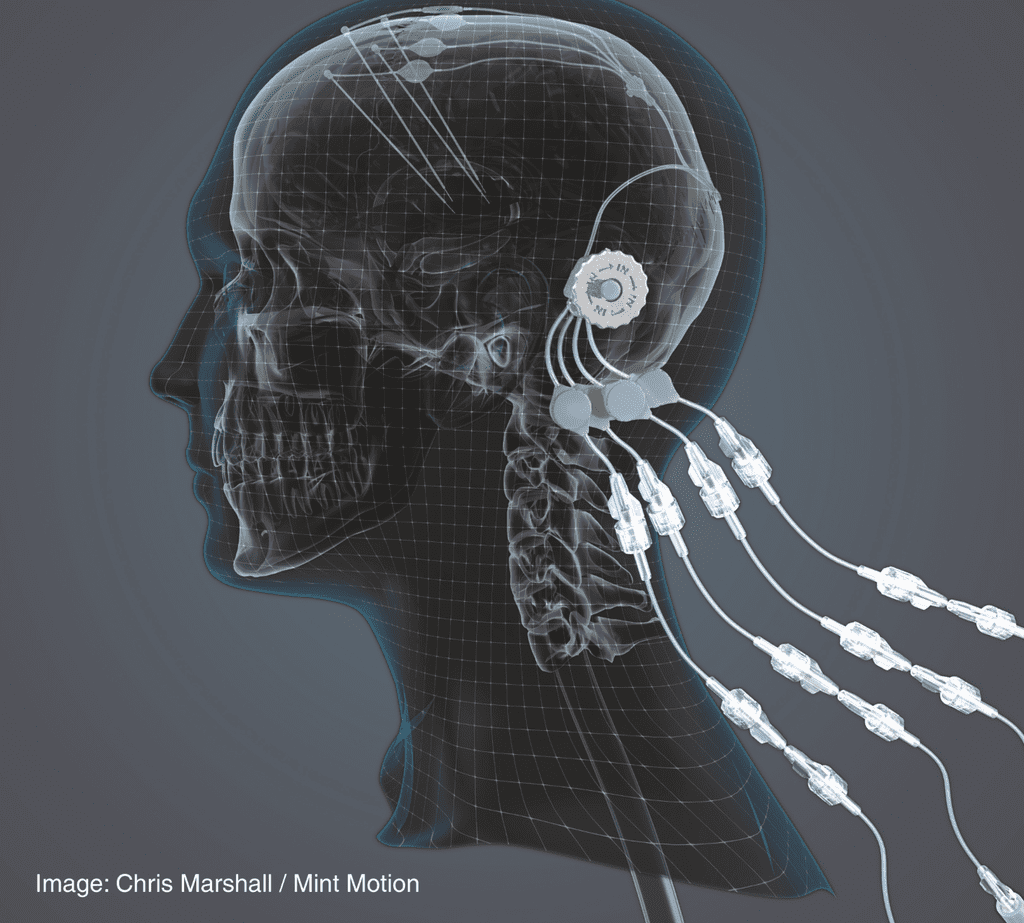
Global engineering company Renishaw has built a device that has been used in a ground-breaking clinical trial. Manufactured by Renishaw on behalf of the North Bristol NHS Trust, the device enabled the precise delivery of a new drug candidate, Glial Cell Line Derived Neurotrophic Factor (GDNF) with the hope of regenerating dying dopamine brain cells in people with Parkinson’s and thereby improve their symptoms.
The results of the trial feature in a two-part BBC Two documentary, The Parkinson’s Drug Trial: A Miracle Cure?
Renishaw worked with Consultant Neurosurgeon, Professor Steven Gill, to manufacture the novel drug delivery system, which offers a practical method to bypass the blood-brain barrier. During the trial, 42 patients had the additively manufactured titanium port embedded into their skull through which GDNF could be delivered via micro-catheters to the putamen, a critical region of the brain for motor function. The device is implanted using the Renishaw neuromate™ surgical robot, which positions four catheters into the brain.
The trial was funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. Results announced on February 27th showed that the drug delivery system performed effectively and reliably and a similar device, developed by Renishaw called ‘neuroinfuse™’, is now being used in other clinical trials.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patient’s brains over months or years through a small implanted port that emerges through the skin behind the ear,” explained Professor Steven Gill. “This is a significant breakthrough in our ability to treat neurological conditions, such as Parkinson’s because most drugs that might work cannot cross from the bloodstream into the brain due to a natural protective barrier.
“Even at a low dose we have seen evidence of patient improvement, which is incredibly encouraging,” added Professor Gill. “Now we need to move towards a definitive clinical trial using higher doses and this work urgently needs funding. I believe that this approach could be the first neuro-restorative treatment for people living with Parkinson’s which is, of course, an extremely exciting prospect.”
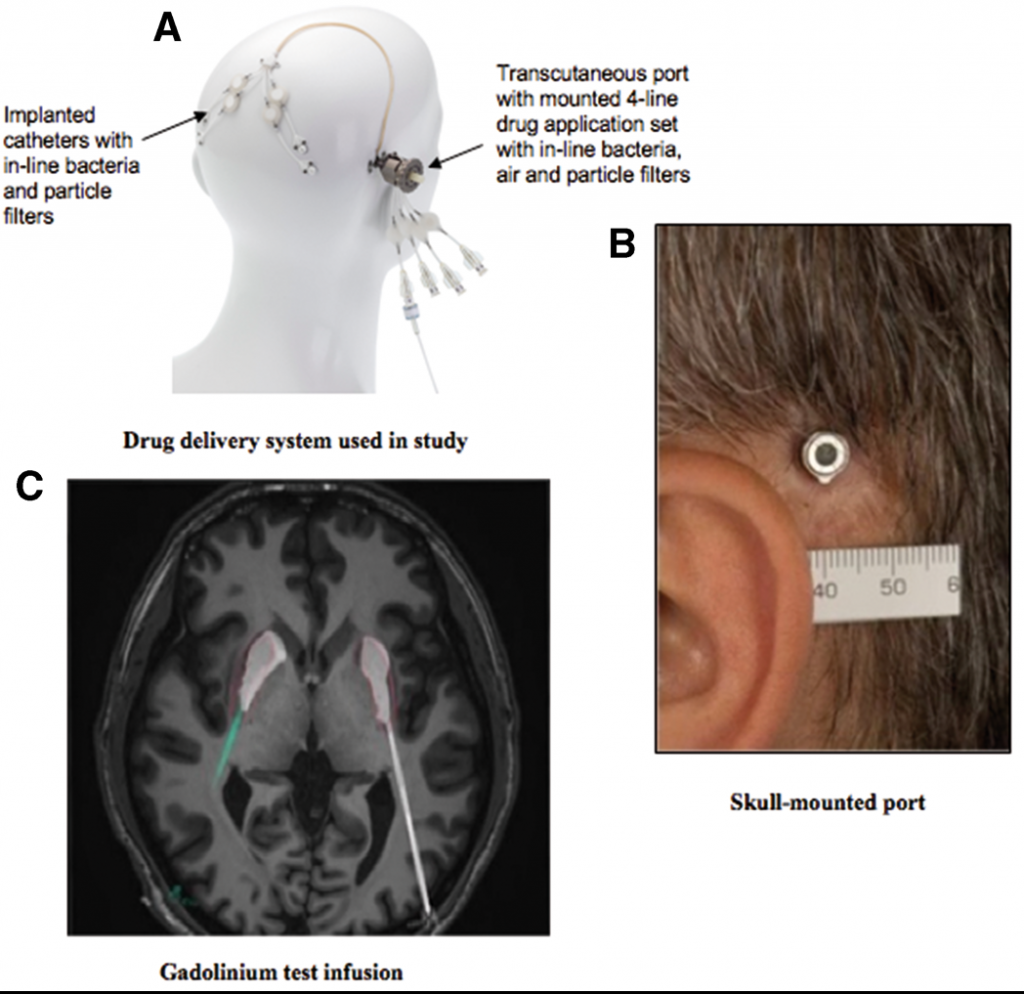 Demonstration of the GDNF delivery device and its position in the skull.
Demonstration of the GDNF delivery device and its position in the skull.
“It has been a privilege to work alongside the study team and with the participants in this ambitious trial,” explained Paul Skinner, General Manager for Neurological Products at Renishaw. “We are very encouraged that there were changes in the brain scans, demonstrating that GDNF is having an effect, and that the delivery system achieved precision administration of drugs into the brain.
“This provides great potential for using the drug delivery system, being developed by Renishaw, for future Parkinson’s studies and experimental treatments for other neurodegenerative diseases and brain tumours.”