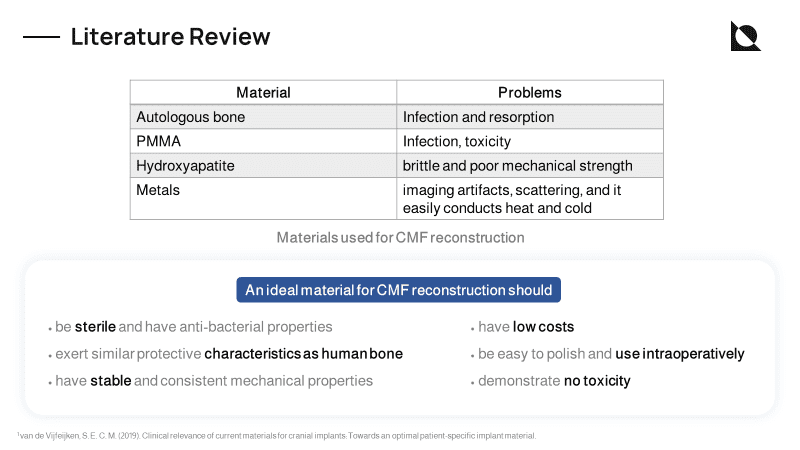
The face is not only the index of the mind but also houses vital organs like the brain and eyes, making treatment of facial injuries a complicated affair. The success of the facial reconstructive procedure is primarily based on the experience and expertise of the surgeon, but a wrong step in treatment can cause lifelong trauma. The reconstruction of a bony contour, discontinuity, or composite defect is generally based on the placement of either a bone graft or the use of an off-the-shelf implant or by using patient-specific implants (PSI).
Patient-Specific Implants (PSI) are now most commonly manufactured using 3D printing technologies like SLM for metals (Ti6Al4V, CoCr), SLS & FDM for polymers (PEEK, PEAK, PCL, PLA), and ceramics (HA, TCP), and SLA for a few advanced ceramics (HA-TCP). But conventionally used materials like metals have huge drawbacks like thermal stability, heavyweight causing discomfort to the patient, and stress shielding.
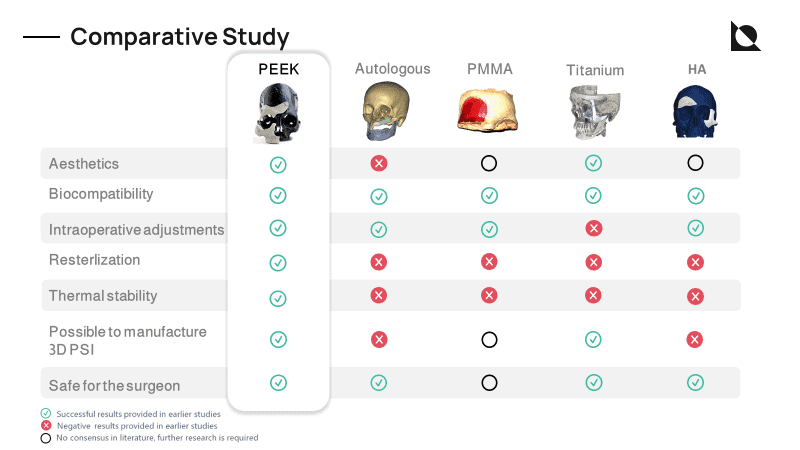
Poly (Ether Ether Ketone) (PEEK) is a high-performance polymer that has high mechanical strength, biocompatibility, and has no cytotoxic activity. It does not induce adverse reactions to human tissues and causes no artifacts in post-operative imaging [1]. Apart from the above-mentioned benefits, PEEK also has mechanical properties similar to that of human cortical bone (thereby reducing stress shielding)
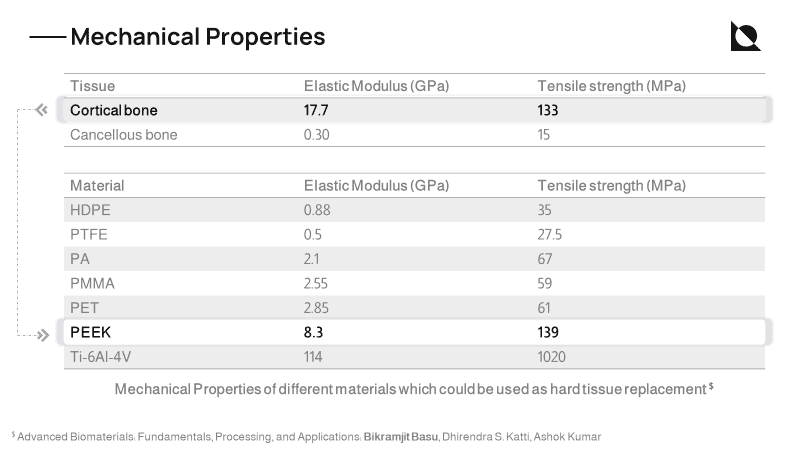
Moreover, PEEK does not conduct temperature like metallic implants.
Process of PEEK Implant Manufacturing

- The surgeon orders a standard diagnostic CT scan of the patient’s affected region. The CT scan is then sent to us through our online surgeon portal.
- The 2-Dimensional patient scan is refined, and a 3D dataset is created for 360-degree visualization. Our engineers use computer-aided design, or CAD, software to design the customized implant and/or surgical guide and/or anatomical model in three-dimensional space, which is further sent to the surgeon for approval.
- Once the virtual design is approved, the anatomical model is fabricated for mock surgical evaluation.
- Once the mock surgical evaluation is done and the implant design is approved, the customized PEEK implant and/or surgical guide is fabricated using Apium M220.
- The implant, along with the anatomical model and/or surgical guide, goes through rigorous quality control stages in compliance with the requirements of the ISO 13485. The cleaning, grinding, sterilization, and packaging of patient-specific implants and surgical guides are carried out in the company’s certified ISO Class 7 cleanrooms.
- After the post-processing and QC check, the implant, along with the model, is shipped to the surgeon or hospital.
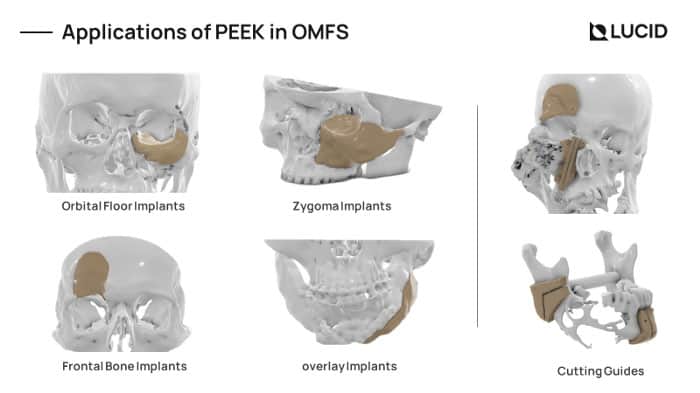
3D printed PEEK Implants is best used for non-loadbearing applications like cranial plates, frontal bone implants, zygomatic implants, mandible and maxilla overlay grafts. Due to its cost-effectiveness, we are also extensively using it for the fabrication of surgical cutting guides for tumor resection.
Case Studies
So far (November 2021) we have positively impacted and transformed over 235 lives with our patient-specific implant solutions. Specifically, with PEEK implants we have done over 25 cases. We anticipate to impact and transform 3500 more lives in the next 18 months. Our success is measured in the patients we reach and the lives we save and improve.
A few case studies:
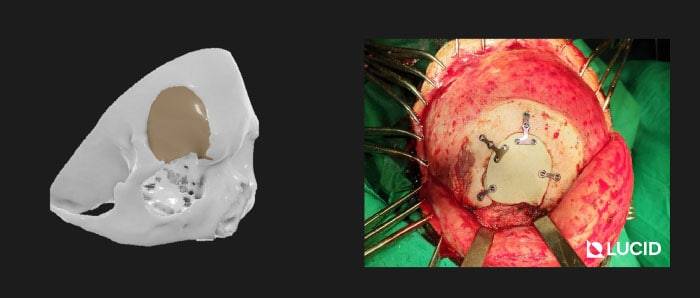
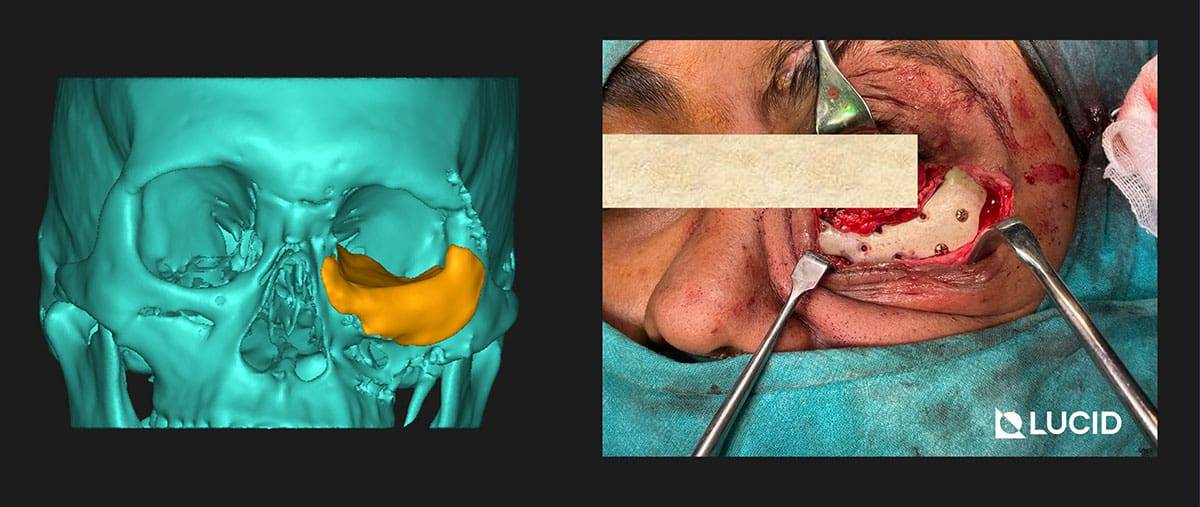
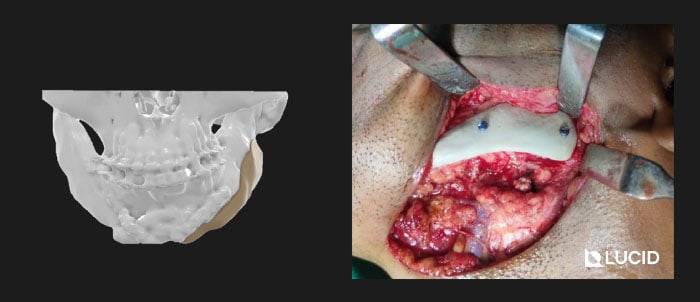
You can find more detailed case studies in our company blog.
Conclusion and future trends
PEEK material as an implant has overcome most of the conventional materials drawbacks and is being considered an ideal reconstructive material.
We see the trend of musculoskeletal reconstructive solutions evolving from metals to bone-like polymers (like PEEK) to actual bone regenerating materials. Our team at LUCID has extensively researched the synthesis of the scaffold material for the 3D plotting of bone grafts. All the required preclinical (lab, animal, and human) studies are done. We are also researching other biomaterials and thermoplastics — PCL, PLGA, and PLA. Our planned commercial launch for bone regenerating implants is in 2022 Q2.
About LUCID Implants
LUCID Implants is a medical technology company established in 2018 in Nagpur, India, which provides customized implant solutions. At LUCID Implants, we design, develop, manufacture and market craniomaxillofacial (CMF) and neurosurgical implants that are individually sized and shaped — what we call customization — to fit each patient’s unique anatomy.

We invite strategic partners, surgeons, and leaders to join us and share their thoughts, suggestions, and feedback. Please write to me at [email protected] for exploring any potential synergies.

