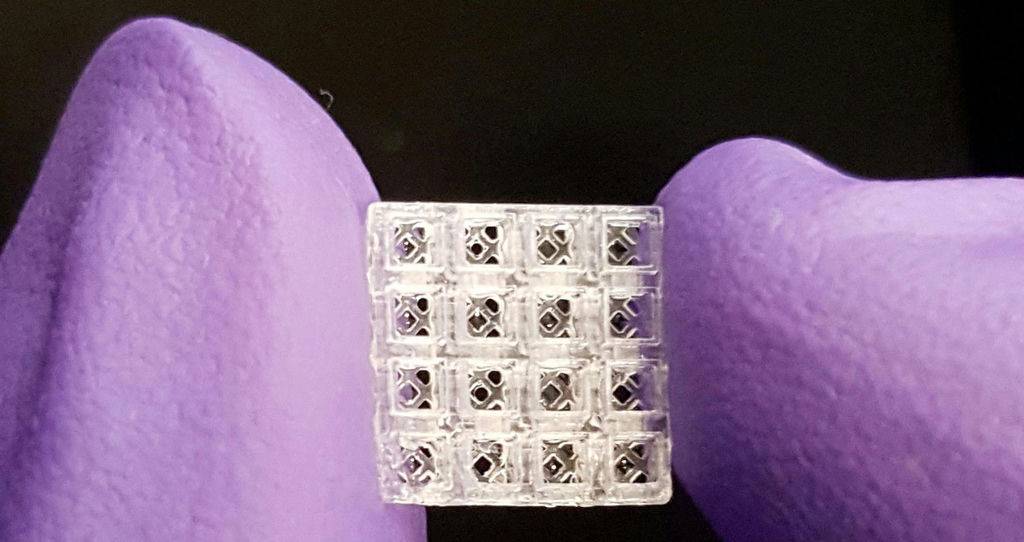
Researchers from Oregon Health & Science University (OHSU) have 3D printed miniature Lego-style ‘bone bricks,’ which are potentially capable of healing broken skeletal tissue.
The researchers’ tiny hollow blocks, which are only the size of a small flea, serve as scaffolding onto which both hard and soft tissue can regrow. Moreover, the stackable nature of the modules enables them to be interlocked like toy bricks, offering scalability along with thousands of potential geometric configurations. Eventually, the Oregon team aims to scale the technology, and use the microcages to produce laboratory-made organs for human transplant instead.
“Our patent-pending scaffolding is easy to use; it can be stacked together like Legos and placed in thousands of different configurations to match the complexity and size of almost any situation,” said Luiz Bertassoni, Ph.D, Associate Professor of Biomedical Engineering at the OHSU School of Medicine.
3D printed biomaterial scaffolds
Printed scaffold biostructures have become an increasingly hot topic of research in recent years, especially for applications within tissue engineering or regenerative medicine. Moreover, advances in 3D printing have enabled patient-specific implantable constructs to be designed in a more scalable way, and in some cases they can now even be produced on-site within hospitals. As a result, assembling these complex tissues no longer requires specialist equipment, which in turn, reduces the lead times associated with implant production.
Nonetheless, the development of the ideal scaffold system has still proved to be elusive, and it remains one of the reasons that the technology hasn’t been more widely-adopted within hospital settings. The ideal tissue support needs to be compatible with defect-specific architectures, but while also allowing the controlled loading of cells, growth factors and hydrogels. Additionally, according to the Colorado team, temporal control of the tissue is essential to the tissue’s ingrowth within the grafted material.
The Oregon team’s 3D printed bone bricks
Conventionally, orthopaedic surgeons repair complex bone fractures by implanting metal rods or plates into the patient, in order to stabilize the bone. It’s only later in the procedure, that bio-compatible scaffolding materials packed with powders or pastes, are used to promote healing. The Oregon team on the other hand, have developed a novel scaffolding system, which precisely places hollow blocks filled with small amounts of growth factor gel, closest to where they are needed.
“The 3D-printed microcage technology improves healing by stimulating the right type of cells to grow in the right place, and at the right time,” explained study co-author Ramesh Subbiah, Ph.D, a postdoctoral scholar in Bertassoni’s OHSU lab. “Different growth factors can be placed inside each block, enabling us to more precisely and quickly repair tissue.”
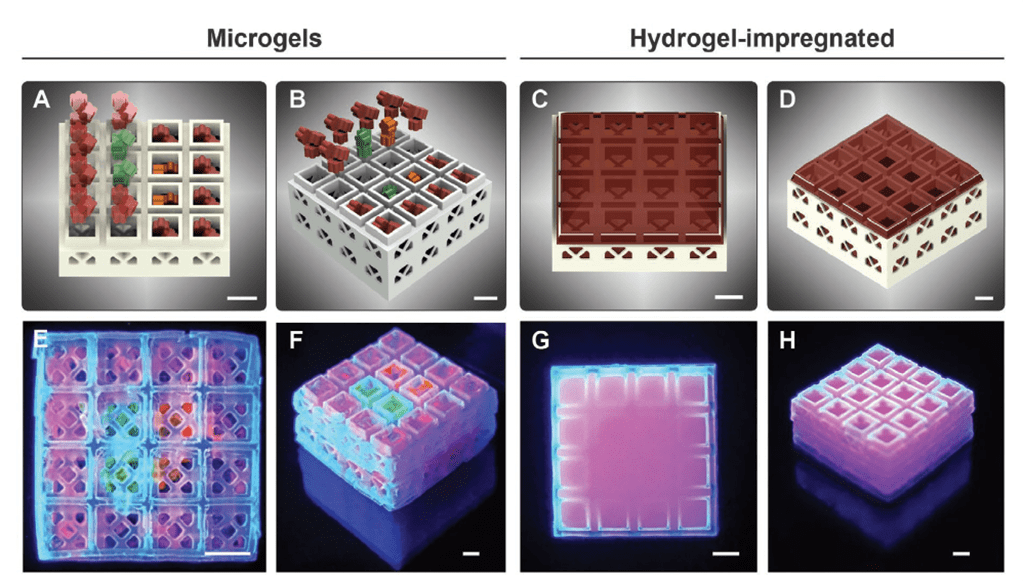
The team’s microcages are hollow on the inside, which enables them to be loaded with a cargo of different bio-gel compositions in a controllable way, and to create scaffolds with spatially defined instructive cues. As a proof of concept, the team 3D printed a number of blocks loaded with microscale granular hydrogels containing various growth factors. Results showed that the cells had entered into the scaffolds, in a quick and controllable manner, thus accelerating the process of new tissue formation and healing.
Testing the researchers’ 3D printed modular design
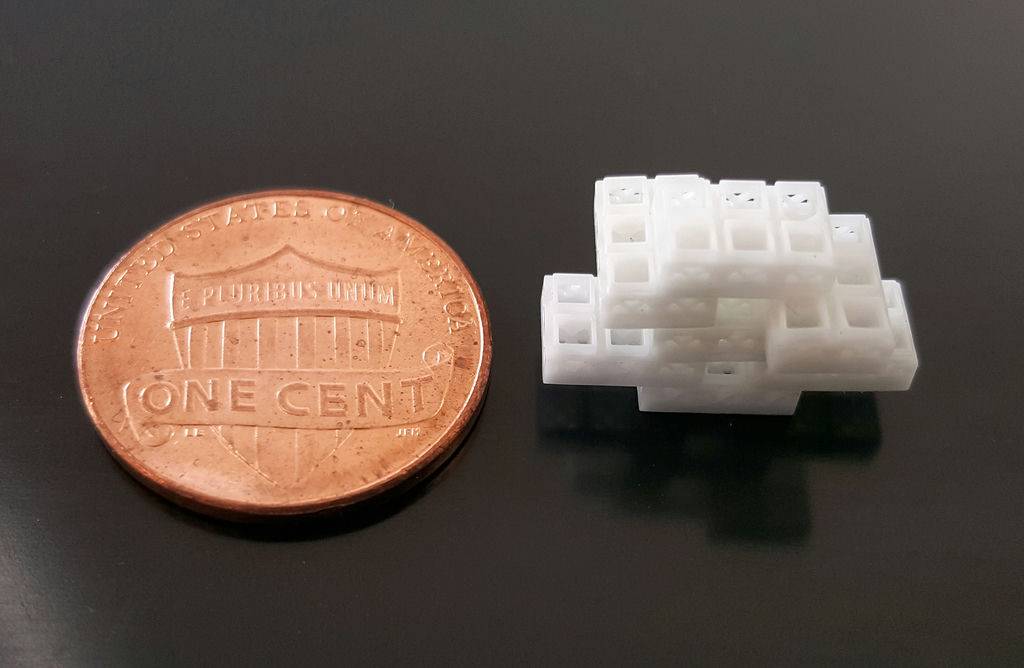
Utilizing a beta-tricalcium phosphate ceramic and a Lithography-based Ceramic Manufacturing (LCM) 3D printing technique, the team created a number of modular miniaturized microcages. The process yielded blocks measuring 3.375 mm3, with hollow dimensions of 1.5×1.5×1.5 mm and a wall thickness of 230–560 µm. Summarily, using the sample bricks, the researchers were easily able to produce various shapes while maintaining a consistent outline across their perimeter.
Working with four layers of 4×4 blocks, the Oregon team calculated that a total of 29,413 configurations were possible, highlighting the potential of the technology for patient-customized bone scaffolds. Illustrating the adaptability of their method for use with other rigid polymeric materials, a number of other bricks were created using a methacrylate-based resin, which is often utilized within similar regenerative procedures.
In order to demonstrate the scaffolds’ potential for regenerative applications, the team used Digital Light Processing (DLP) 3D printing to create a series of shaped products including a five-pointed flower-like geometry. Different combinations of human recombinant growth factors were then manually loaded into the modules within the various shapes. After stacking, the strength of two-layered blocks was significantly reduced to13.8 MPa, but this was still much higher than that reported for the average jaw bone of 3.9 MPa.
What’s more, further experiments found that growth factor-filled blocks placed near repaired rat bones led to around three times more blood vessel growth than conventional scaffolding material. As a result, the researchers concluded that although their method had been optimized for the repair of hard tissues, the concept may be applicable to other tissue regeneration applications. With significantly more research, the Oregon team believes that the modular approach could be used to repair more complex bone fractures in larger animals, or even to make organs for human transplant.
Additive manufacturing and bone repair
The concept of 3D bioprinting bone implants is already being explored by a number of researchers from academic institutions around the world. Researchers from the University of Manchester for instance, have developed a similar bone brick to that of the Oregon team. The device was created in response to the need for urgent medical care in Syrian refugee camps.
Researchers from the Delft University of Technology meanwhile, have designed and printed a porous titanium bone implant with antibacterial properties. The graft’s synergistic antibacterial behavior could give rise to a new type of implant that outlives patients with minimal maintenance.
Scientists from Texas A&M University on the other hand, have combined 3D printing, biomaterial engineering and stem cell biology to create new, more efficient facial bone grafts. The highly-osteogenic scaffolds not only facilitate bone cell growth, but also serve as a sturdy platform for bone regeneration.
In order to develop and evaluate the technology, the Oregon team partnered with colleagues from OHSU, The University of Oregon, New York University and Mahidol University in Thailand. The researchers’ findings are detailed in their paper titled “3D Printing of Microgel‐Loaded Modular Microcages as Instructive Scaffolds for Tissue Engineering,” published in the Advanced Materials journal.
The report was co-authored by Ramesh Subbiah, Christina Hipfinger, Anthony Tahayeri, Avathamsa Athirasala, Sivaporn Horsophonphong, Greeshma Thrivikraman, Cristiane Miranda França, Diana Araujo Cunha, Amin Mansoorifar, Albena Zahariev, James M. Jones, Paulo G.Coelho, Lukasz Witek, Hua Xie, Robert E. Guldberg and Luiz E. Bertasson.